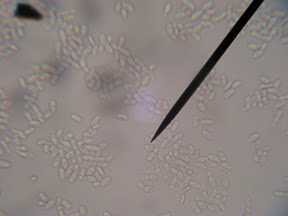
This was sort of the catalyst for the whole blog. I ran across
written by Dave Whitman titled "Sterile Distilled Water Yeast Storage". In this paper, Dave details pretty much everything I have going here. Instead of just regurgitating everything Dave wrote in my own words I'll just repost his article here for archival purposes (as he grants this at the bottom of his article) and add my notes before.
I will attest that, this method works. How long it works I guess is up to your lab habits. I've been able to isolate and store over 6 strains of yeast in 1 dram vials and take those and reanimate them in clean test tubes. For the sterile water, I am using generic saline solution that you would generally use for contact lenses.
So bravo Dave for writing this! Your work is well appreciated.
Sterile Distilled Water Yeast Storage
v1.0 10/1/96 by Dave Whitman (dwhitman@rohmhaas.com)
INTRODUCTION
Yeast can be stored for long periods of time (months to years) in a dormant state under sterile distilled water. The samples are tiny, so you can keep many different yeast strains in a small area. No refridgeration is needed.
The procedures I use are largely adapted from a 1 day course in yeast propagation that I took at the American Type Culture Collection. ATCC is in the Washington DC area, and they give the course once a year; I recommend it highly if you're within driving distance. There is a very nominal fee ($30?), and you come away with free yeast cultures to offset the cost of the course.
STERILE TECHNIQUE
Be fanatical about sanitation. You'll be transfering very small numbers of yeast cells around, and the tiniest amount of bacterial contamination can force you to re-isolate a culture.
Wort, distilled water, and all containers should be STERILIZED (not just sanitized). This means pressure cooking 20 minutes at 15 psi, or repeatedly boiling for 30 minutes on 3 days in a row.
I sterilize my innoculation loop by heating it in a flame until it turns red, then quench it by touching sterile water or the surface of a slant. Alternatively, you can use sterile flat toothpicks - wrap a bunch in aluminum foil and pressure cook along with everything else. Another option is to use disposable sterile syringes.
Work in a draft free room to avoid stirring up dust. Avoid using a vacuum cleaner in the same house for several hours before, because it will stir up dust. Wipe down your work surface with bleach diluted 10:1 just before starting. Follow that with a alcohol wipe down. Open and close containers quickly, while trying to hold the opening horizontal (to minimize the chance of dust drifting in).
Arrange everything in an organized way on your work surface so that you don't have to move your hands around too much and generate air currents.
PREPARATION OF SAMPLES FOR STORAGE
Start with yeast cultures growing on slants or plates.
Add 2-3 ml distilled water to small vials. I use 1 or 2 dram vials, readily available at places that sell essential oils or bulk perfumes. Pressure cook the loosely capped water vials for 20 minutes at 15 psi.
Using a sterile loop or other implement, grab a small amount of solid yeast from a slant. You don't need or want too much - about the size of a match head. Try to avoid picking up any of the solid media. Transfer to a water vial, then cap it tightly and wrap the cap with tape to seal. I like to make 2 vials of each yeast. One is "working", the other is "archival".
You can store these vials at room temperature for at least 6 months, probably for years. The concept is that in distilled water with no nutrients around, the yeast just go dormant. (That's why you want to avoid transfering any nutrient media when you grab the sample - you're TRYING to starve them).
PREPARATION OF STARTERS FROM STORED SAMPLES
I make up premeasured samples of starter wort ahead of time: SG 1040 wort, pressure canned in containers of the right sizes to build up a reasonable starter volume in safe steps. I use a 3-step build up: 4 mls of wort in ascrewcap test tube, 40 ml of wort in a 10 oz juice bottle, and 350 ml of wort in a 16 oz juice bottle.
The nice thing about using old juice bottles is that they have vacuum seal caps. Put the caps on loosely, pressure cook 20 minutes at 15 psi, then tighten lids while the wort is still fairly hot. As they cool, the vacuum seal should form. The test tubes don't have vacuum seal lids, but if you tighten them and tape the joint, they seem to keep well. After sealing, shake up the samples to pre-aerate them using the air in the head space of the container.
I start building my starter up 1 week before brewing. Shake up a "working" vial to resuspend the yeast, then open it and quickly transfer a single drop of suspension into a test tube with 4 ml of wort using a sterile tool such as aninnoculation loop. Reseal the vial and loosely cap the test tube. Because you use only one drop, as long as your sanitation is good the stored culture can be used for many, many batches.
Let the yeast work for 1-2 days, swirl and dump the whole thing into a 40 ml wort sample. Don't expect to see much of any activity at either of these stages - there just isn't enough yeast to generate any obvious CO2 evolution. After another 1-2 days, swirl, then dump the whole 40 ml into a 350 ml wort sample. After 1 or 2 days, you should see visible bubbling in this larger starter. You could (and arguably should) continue to step this up to even higher volume, but for ales I get good results just dumping the 350 ml starter into a 5 gallon batch.
I don't bother with an air lock for any of the starter stages - I just keep the lid on loosely to allow CO2 to escape. I haven't had a starter go bad yet after about 10 batches. YMMV.
LONG TERM CULTURE MAINTANENCE
Periodically, it's a good idea to reculture the yeast to ensure that they're alive and healthy. At the ATCC course, they recommended reculturing every 6 months, although they said there was literature precident for storing samples for 5 years. For comparison, they said that if you use slants for storage, you should reculture every 2-3 months. I suspect many people keep slants far longer than this.
They also recommended only saving samples in water that you pull off of solid media like a slant or plate, since you can look at it and see if there is any obvious contamination.
To reculture, prepare slants. Open your "archival" (unused, and hence uncontaminated) vial, and streak a single drop of slurry onto one or more slants. Let them grow out, and assuming the colonies looks clean and healthy, transfer to sterile distilled water for another storage period.
SLANT PREPARATION
Prepare a small amount of SG 1040 wort. Add 1.5% agar by weight, and heat until the agar dissolves. While still hot, add 2-3 ml of solution to 2 dram vials, then loosely cap. Put the vials in a tin can or something to hold them upright, then pressure cook 20 minutes at 15 psi. While still hot, tighten the caps, then prop the can at an angle to tilt the vials at a 45 degree or greater angle. Allow to cool and solidify.
You can buy agar cheaply at oriental groceries. In my area, it comes in packages of 1" square sticks that are about 10" long. There are 2 sticks to a package - one is off-white, the other is dyed lurid red which makes it easy to spot on in the store. The sticks have the texture of styrofoam, and you can easily break off or cut little pieces for making slants.
 If your only experience with yeast is brewing beer, you're missing the point. Yeast is one of the greatest gifts on this planet. Wine, Beer, Rum, Whiskey... Bread. All of these things do not exist without yeast. Man alone can not create alcohol... and in this post, man alone cannot create good bread without yeast.
If your only experience with yeast is brewing beer, you're missing the point. Yeast is one of the greatest gifts on this planet. Wine, Beer, Rum, Whiskey... Bread. All of these things do not exist without yeast. Man alone can not create alcohol... and in this post, man alone cannot create good bread without yeast. Place dough into the greased loaf pan and place in a warm place until the dough has risen to the level you are satisfied with (hour or two). Bread (yeast based) will not rise anymore than it has already risen when placed into an oven. That process would require baking powder and that is another post that you can find on another blog. So, the state that it is in when you put it in the oven is the state it will be in when it comes out (more less).
Place dough into the greased loaf pan and place in a warm place until the dough has risen to the level you are satisfied with (hour or two). Bread (yeast based) will not rise anymore than it has already risen when placed into an oven. That process would require baking powder and that is another post that you can find on another blog. So, the state that it is in when you put it in the oven is the state it will be in when it comes out (more less).